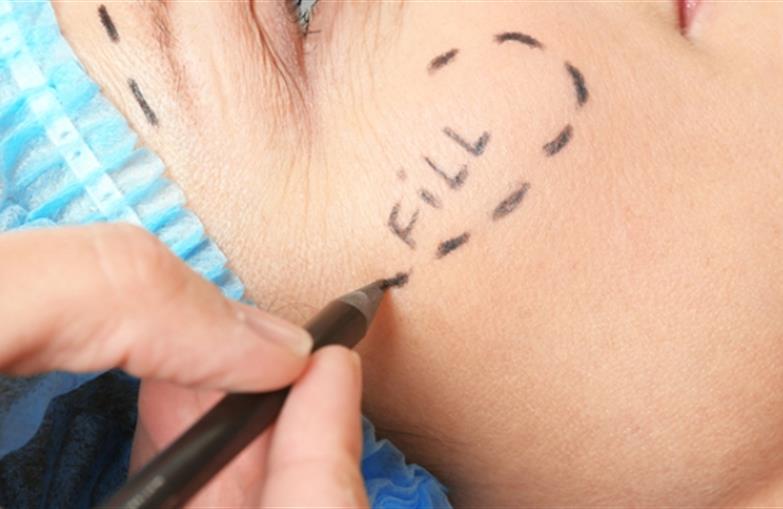
In a recent safety communication, the Agency warned that handheld devices should not be used to perform aesthetic filler procedures as these “pens” use high pressure to force substances into the body and do not provide enough control over where the injected product is placed. Dermal fillers approved by the FDA are intended for use only with a syringe with a needle or cannula and are supplied by prescription.
Reports of needle-free devices, along with the lip and facial fillers, being sold online to consumers and promoted through social media channels prompted the FDA alert. These unapproved products may be contaminated with chemicals or infectious organisms that could potentially result in severe complications.
Adverse events experienced with these devices and products should be reported to the FDA’s MedWatch program. Updates will be provided by the Agency as new information becomes available.